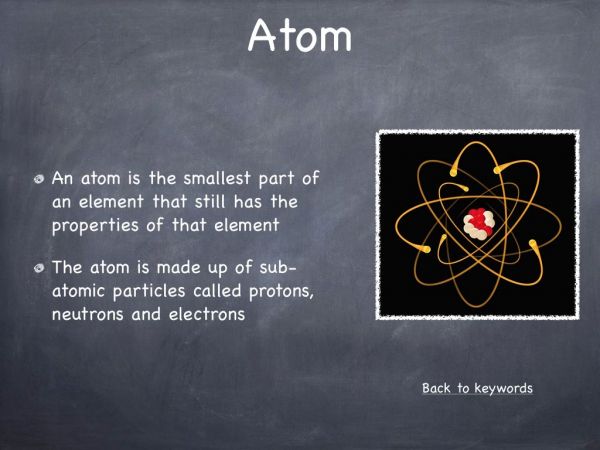
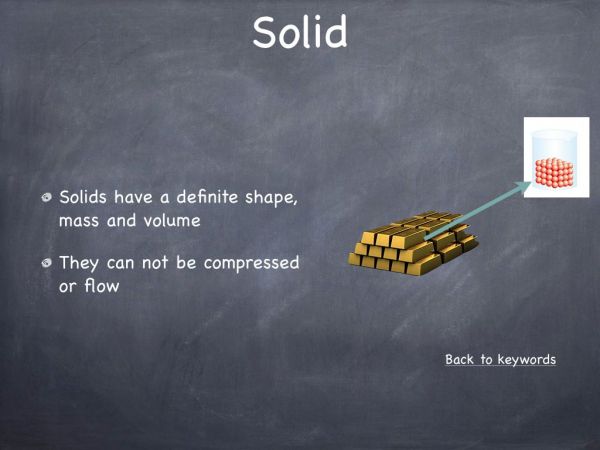
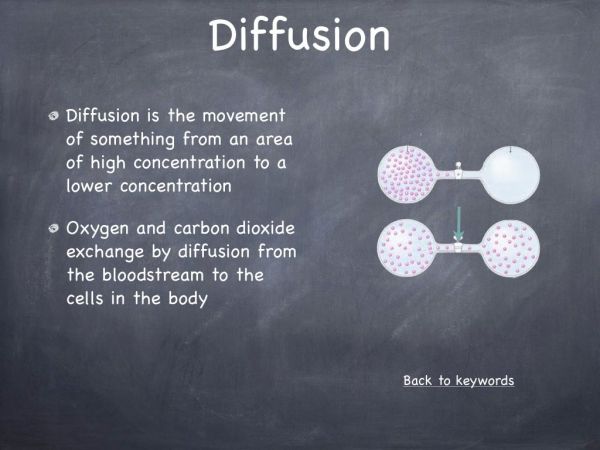
The majority of students will know different examples of acids and bases. A common misconception amongst students is that acids are dangerous and corrosive, for example stomach acid (hydrochloric acid) while bases are noncorrosive and not harmful. In this video we aim to display how dangerous and corrosive bases can be.
Video: Aluminium dissolving in NaOH
Methodology
Materials
- 2 aluminium cans
- Steel wool
- 2 beakers
- Sodium hydroxide solution (~6M)
- Metal tongs
- Stirring rod
Method
Scrub one aluminium can with steel wool to remove the plastic coating. Leave the other can, as is, with the plastic coating intact. Place the two cans in two separate beakers. Pour concentrated sodium hydroxide solution into and around each can in a well ventilated area. Leave for a few minutes and observe the decomposition of the cans.
Tips
Pierce the can without the plastic coating to increase the base’s access to the aluminium. This speeds up the corrosion.
*Precautions/Safety
Theory behind the hook
The aluminium in the can reacts with the sodium hydroxide and water to form sodium aluminium oxide which is soluble in water. Hydrogen gas is also produced:
2 Al + 2 NaOH + 2 H2O → NaAlO2 + 3 H2
Aluminum + Sodium hydroxide → Sodium aluminum oxide + Hydrogen
The can without the plastic coating on the outside corrodes much faster than the can with the plastic coating intact. What is left at the end of the demonstration is the plastic that lines the inside of the can. The plastic is necessary in these cans as soft drinks like coke are mildly acidic and the aluminium would dissolve in the solution over time. Aluminium is an amphoteric substance (will react with both an acid and a base). This video can also be used to display that plastics are extremely strong and are very hard to breakdown.
How this hook works
This hook is effective as it provides a very visual demonstration of the corrosive power of strong bases. The demonstration takes around 25 mins and could be set up at the start of the lesson and revisited later. Students could be asked to discuss and make prejudgments about the power of acids and bases. In this way misconceptions can be addressed in class. This is a very visual representation of what a corrosive base can do and also aid in the explanation of the term “corrosive”.
Questions & Answers
- Why does one can corrode faster than the other? Are they different cans? What is the difference?
The plastic protects the can which slows down the decomposition of the aluminium. - What does this demonstration tell us about plastics?
This tells us that some plastics are extremely hardwearing as it takes longer for the can with plastic to corrode in comparison to the aluminium can. - Where does the aluminium go? Does it simply disappear?
No, it changes into sodium aluminium oxide which is soluble in water. This gives the solution the lack colour at the end of this demonstration. - What would happen if a weak base was used?
In theory the same reaction would take place but it would take a great deal longer, days, weeks or maybe even years.